In the global fight against the COVID-19 pandemic, vaccines have emerged as potent tools in curbing the spread of the virus and reducing severe illness. Among the frontrunners is the Covishield vaccine, developed by AstraZeneca in collaboration with the University of Oxford. However, recent admissions by AstraZeneca regarding rare side effects associated with the vaccine have stirred public concern and ignited discussions on vaccine safety. In this blog post, we delve into the details of these revelations, explore the implications for public health, and discuss strategies for addressing public apprehensions.
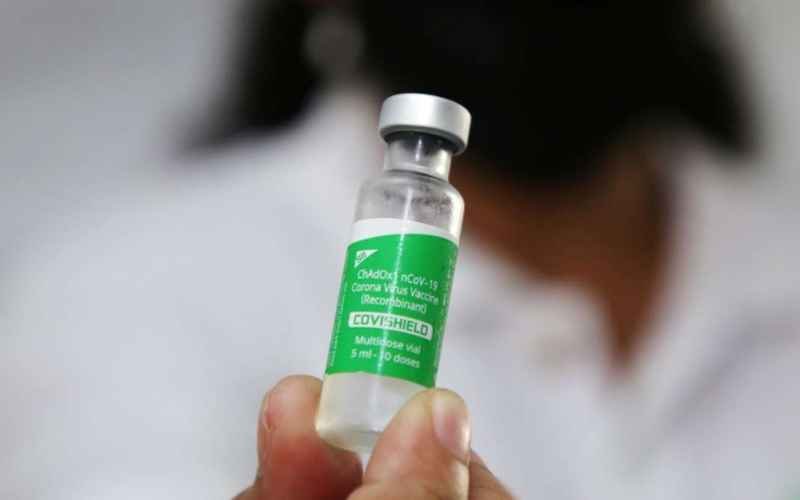
Understanding the Rare Side Effect: AstraZeneca has acknowledged the rare occurrence of blood clotting disorders, particularly cerebral venous sinus thrombosis (CVST), in individuals who have received the Covishield vaccine. While the incidence of these events is extremely low, ranging from one in several thousand to one in several hundred thousand vaccinated individuals, their potential severity warrants careful attention. CVST involves the formation of blood clots in the brain’s venous sinuses, leading to potentially life-threatening complications.
Public Reaction and Concerns: The acknowledgment of rare side effects by AstraZeneca has understandably triggered concerns among the public. Questions regarding the safety and efficacy of the Covishield vaccine have been raised, with some individuals expressing hesitancy towards vaccination. The dissemination of information, both accurate and sensationalized, through various media channels has contributed to the amplification of these concerns, creating a challenging landscape for public health authorities and healthcare professionals.
Navigating Public Concern: Addressing public concerns surrounding vaccine safety requires a multifaceted approach centered on transparency, education, and risk communication. AstraZeneca’s transparency in acknowledging the rare side effects is a crucial first step towards building trust and credibility. It is imperative for public health authorities and healthcare providers to disseminate accurate information about the benefits and risks of vaccination, emphasizing the overwhelming evidence supporting the safety and efficacy of vaccines in preventing severe COVID-19 illness and reducing transmission.
Enhanced Surveillance and Monitoring: Robust surveillance systems play a pivotal role in detecting and assessing adverse events following immunization. Healthcare professionals should remain vigilant for any signs or symptoms suggestive of blood clotting disorders in individuals who have received the Covishield vaccine. Timely reporting of such events facilitates thorough investigation and allows for the implementation of appropriate risk mitigation strategies.
Individualized Risk-Benefit Assessment: While the overall benefits of vaccination outweigh the risks, individualized risk-benefit assessments may be necessary, particularly for populations with specific risk factors for thrombotic events. Healthcare providers should engage in open and honest discussions with their patients, weighing the potential risks and benefits of vaccination based on individual circumstances and medical history.
Conclusion: The acknowledgment of rare side effects associated with the Covishield vaccine underscores the importance of ongoing vigilance and transparency in vaccine safety monitoring. Navigating public concern requires a collaborative effort involving pharmaceutical companies, public health authorities, healthcare providers, and the media. By prioritizing transparency, education, and risk communication, we can foster confidence in vaccination and continue our collective efforts to combat the COVID-19 pandemic.
Related posts:
 Man Receives Over 200 Covid-19 Vaccinations, Study Finds No Immune Fatigue
Man Receives Over 200 Covid-19 Vaccinations, Study Finds No Immune Fatigue
 Study Finds Elevated Risk of Rheumatic Disease for Up to One Year Following Covid Infection
Study Finds Elevated Risk of Rheumatic Disease for Up to One Year Following Covid Infection
 Understanding Insulin: Who, How, When, and Why
Understanding Insulin: Who, How, When, and Why
 चमकदार त्वचा के लिए सरल और प्राकृतिक तरीके
चमकदार त्वचा के लिए सरल और प्राकृतिक तरीके
 Remembering Rinky Chakma: Former Miss India Tripura 2017, A Beacon of Beauty and Resilience
Remembering Rinky Chakma: Former Miss India Tripura 2017, A Beacon of Beauty and Resilience
 From Flushing Kidney Stones to Boosting Creativity: 5 Surprising Health Benefits of Beer
From Flushing Kidney Stones to Boosting Creativity: 5 Surprising Health Benefits of Beer
 Effect of Cancer on Children vs Adults: Understanding Leukaemia, challenges and treatments
Effect of Cancer on Children vs Adults: Understanding Leukaemia, challenges and treatments
 Cherries to Bananas: 5 Superfoods to Help You Get a Restful Night of Sleep
Cherries to Bananas: 5 Superfoods to Help You Get a Restful Night of Sleep
 Navigating Rheumatoid Arthritis and Preeclampsia During Pregnancy
Navigating Rheumatoid Arthritis and Preeclampsia During Pregnancy
